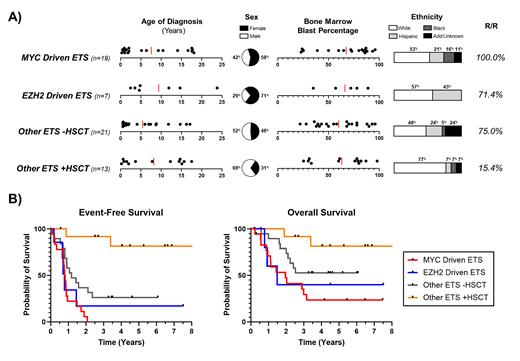
Pediatric Acute Myeloid Leukemia (pAML) patients with a fusion involving an E26 transformation-specific (ETS) transcription factor have traditionally been considered high-risk. The most common fusions include ETV6::MNX1 t(7;12)(q36;p13) and FUS::ERG t(16;21)(p11;q22), yet primary fusion alone has proven incapable of predicting response to treatment. Indeed, within the 60 pAML patients with ETS fusions from the Children's Oncology Group (COG) trials AAML0531 and AAML1031, there were 25 unique fusions and diverse outcomes between patients who did share a primary fusion. A molecular based reclassification of ETS fused pAML could prove beneficial in the development of novel therapeutic additions to standard induction. Here, using genetic and RNAseq data from over 1,400 pediatric patients from AAML0531 and AAML1031, we reclassified ETS patients based on oncogenic transcriptional profiles as well as access to hematopoietic stem cell transplant (HSCT) ( Figure 1A).
(19/60) patients presented with an enhanced MYC transcriptional signature (p-val < 0.00) independent of fusion partners [EP300::ETV6, ETV6::CCND3, ETV6::FAM133B, ETV6::FOXO1, ETV6::INO80D, ETV6::LMBR1, ETV6::MNX1, EWSR1::FEV, FLI1::IFIT2, FUS::ERG, FUS::FEV, FUS::FLI1]. Median event-free survival (EFS) was 293.5 days; median overall survival (OS) was 620.5 days. Notably, 100% (19/19) of patients with a MYC-driven signature relapsed; of the six patients who received HCST, five died within 3 years of diagnosis. Preliminary in vitro data in ETS cell lines with an enhanced MYC transcriptional signature (YNH-1, TSU-1621) suggests that the BET bromodomain inhibitor JQ1 may dampen MYC and induce anti-proliferative effects.
(7/60) patients were defined by an EZH2-driven “immune-cold” signature characterized by global loss of immune receptors. Patients presented at diagnosis with significantly elevated EZH2 and low levels of MHC I and II receptors typically observed at relapse; additionally, there was significant downregulation in T cell and natural killer cell ligands and a 100% failure rate for HSCT. Median EFS was 255 days and median OS was 339 days. We have previously discussed the mechanism of leukemogenesis as well as proposed therapeutic interventions for this subset (Buteyn, ASH 2021).
(34/60) patients did not possess either a MYC-driven or EZH2-driven signature. These “other” ETS were instead defined by whether or not the patient underwent HCST. (21/34) patients did not receive HCST and had median EFS and OS of 429 and 1194 days, respectively. The relapsed or refractory rate in this population was still 75%; (8/14) of R/R patients were deceased within three years. The remaining (13/60) ETS patients did receive HSCT in first remission. (11/13) are still alive and at a rate higher than the general pAML population post-HSCT (62.6%). Despite a survival rate at approximately 85%, ‘Other ETS +HSCT’ patients were all still categorized as ‘High Risk’ at the end of AAML1031.
Overall, reclassification based on molecular drivers appears to not only be more accurate in predicting relapse and final outcome, but also in proposing novel agents for pAML patients who overwhelmingly do not respond to standard regimens. Reinvigoration of immune recognition by inhibition of EZH2 and/or inhibition of MYC-driven proliferation via BET inhibitors may prove an essential step in setting the stage for successful HSCT for a significant portion of pAML ETS patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal